Today, the U.S. Food and Drug Administration announced it is investigating consumer complaints of Cronobacter sakazakii and Salmonella Newport infections.
All of the cases are reported to have consumed powdered infant formula produced from Abbott Nutrition's Sturgis, Michigan facility.
As a result of the ongoing investigation, along with the U.S. Centers for Disease Control and Prevention and state and local partners, the FDA is alerting consumers to avoid purchasing or using certain powdered infant formula products produced at this facility.
This is an ongoing investigation, and the firm is working with the FDA to initiate a voluntary recall of the potentially affected product.
The FDA is advising consumers not to use Similac, Alimentum, or EleCare powdered infant formulas if:
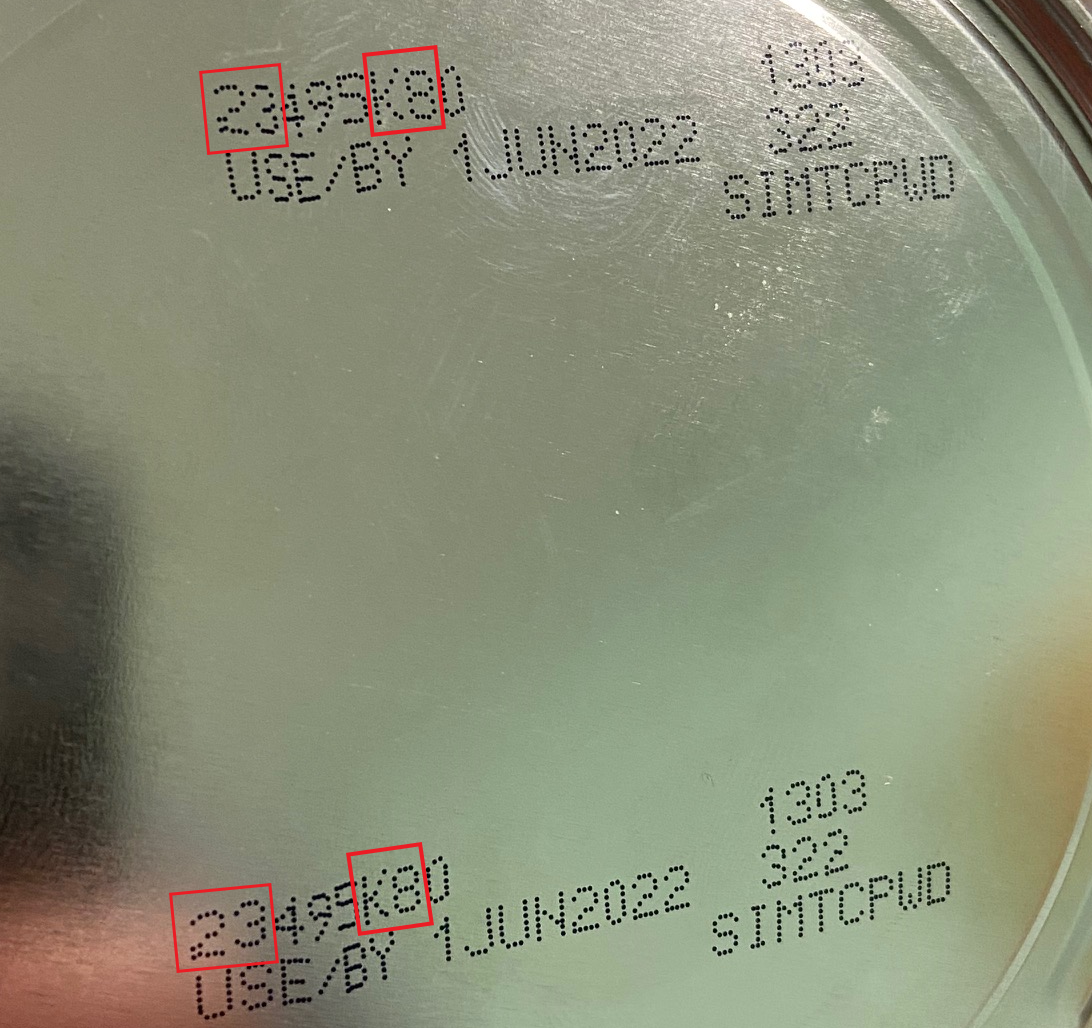
- the first two digits of the code are 22 through 37; and
- the code on the container contains K8, SH, or Z2; and
- the expiration date is 4-1-2022 (APR 2022) or later.

Products that do not contain the information listed above are not impacted. The FDA advisory does not include liquid formula products or any metabolic deficiency nutrition formulas. Consumers should continue to use all products not covered by the advisory.
The FDA is investigating complaints of four infant illnesses from three states. All four cases related to these complaints were hospitalized and Cronobacter may have contributed to a death in one case.
The FDA has initiated an onsite inspection at the facility. Findings to date include several positive Cronobacter sakazakii results from environmental samples taken by the FDA and adverse inspectional observations by the FDA investigators.
A review of the firm's internal records also indicates environmental contamination with Cronobacter sakazakii and the firm's destruction of products due to the presence of Cronobacter.
"As this is a product used as the sole source of nutrition for many of our nation's newborns and infants, the FDA is deeply concerned about these reports of bacterial infections," said Frank Yiannas, FDA Deputy Commissioner for Food Policy and Response. "We want to reassure the public that we're working diligently with our partners to investigate complaints related to these products, which we recognize include infant formula produced at this facility, while we work to resolve this safety concern as quickly as possible."
Parents and caregivers of infants who have used these products and are concerned about their child's health should contact their child's health care provider.
If your child is experiencing any of these symptoms, you should notify your child's healthcare provider and seek medical care for your child immediately.
The FDA continues to investigate and provide additional consumer safety information when it becomes available.
