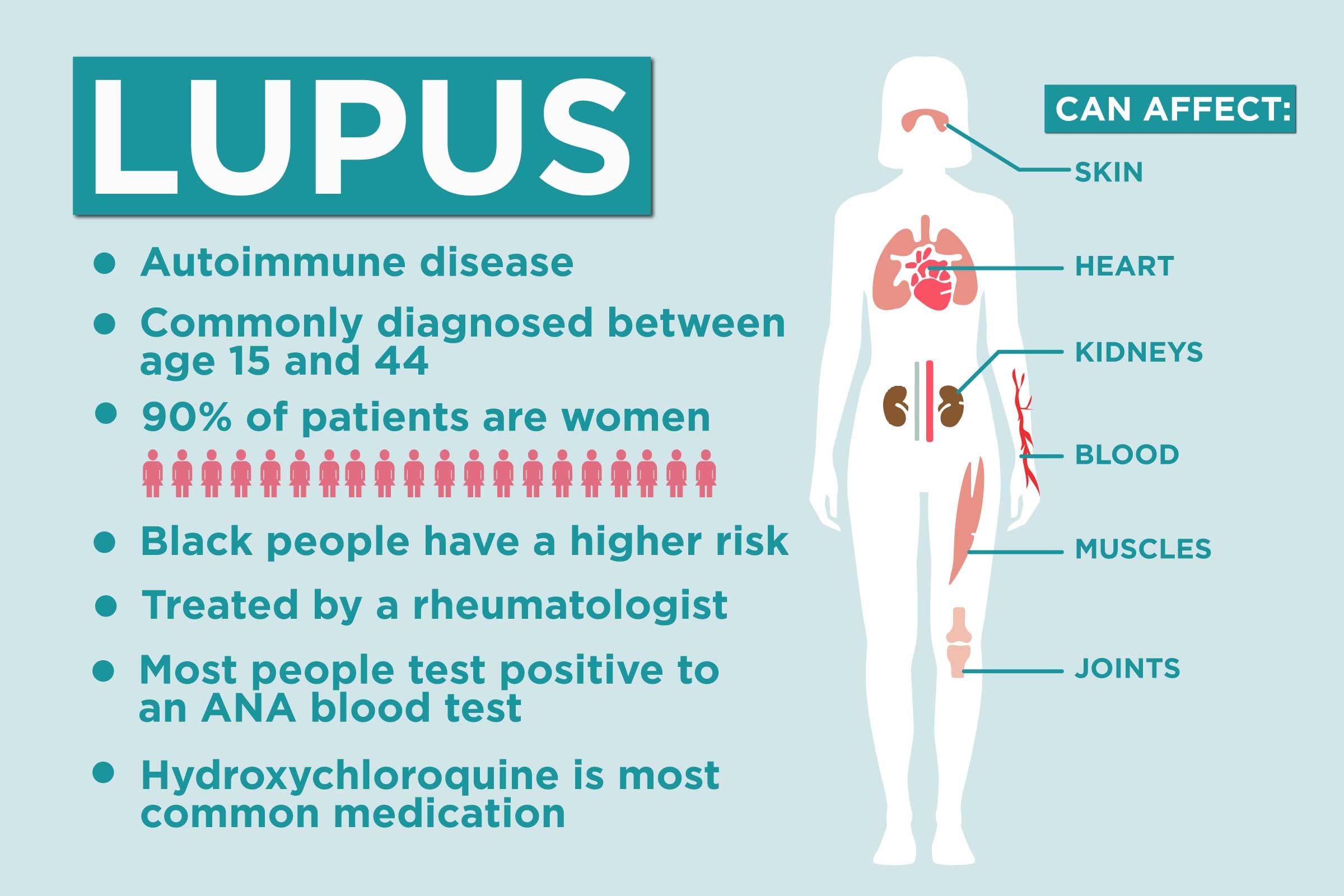
If you have systemic lupus erythematosus (SLE), the most common form of lupus, you likely know that this autoimmune disease can impact many parts of your body, including your skin, joints, and heart.
Kidney damage is another common complication, yet until recently, there wasn’t much you could do to treat lupus-related kidney damage besides eventually go on dialysis. That’s changed, however, thanks to the approval of two new medications.
Most recently, on January 22, the U.S. Food and Drug Association (FDA) approved voclosporin (Lupkynis), making it the first-ever oral drug to treat lupus-related kidney inflammation (medically termed lupus nephritis).
The drug’s manufacturer, Aurinia Pharmaceuticals, submitted data to the FDA from phase 2 and phase 3 clinical trials that tested the safety and efficacy of voclosporin in 533 adults with lupus nephritis. Half of the study participants received 24 mg of the drug twice a day; the other half received a placebo twice a day.
Both groups continued with the standard of care (their usual lupus medications as well as mycophenolate, a disease-modifying antirheumatic drug that is often given to lupus nephritis patients).
After a year, the study participants who had been taking voclosporin were more than twice as likely as those in the placebo group to achieve a “complete renal response,” which means that the level of protein in their urine had returned to normal or near-normal.
Voclosporin works by blocking calcineurin, a substance related to inflammation. Possible side effects include diarrhea, headache, fatigue, and increased blood pressure.
This news follows the FDA approval of belimumab (Benlysta) for lupus nephritis in December 2020, when it became the first drug ever to be approved specifically for lupus nephritis. Belimumab, which is manufactured by GlaxoSmithKline, was approved in 2011 for the treatment of lupus but not specifically for lupus nephritis.
Belimumab, which is taken via IV or subcutaneously, is a biologic drug that inhibits immune system cells called B cells.
Kenneth M. Farber, president and chief executive officer of the Lupus Research Alliance, told Healio Rheumatology that his group “could not be more excited” about the approval of voclosporin.

“Several months ago, there were no new treatments approved specifically for lupus nephritis, and with this announcement, there are now two,” he said, adding that having multiple options will hopefully allow patients to find one that works for them.
In a press release about the approval of Lupkynis, Kathleen A. Arnsten, President and CEO of Lupus and Allied Diseases Association, who is herself a lupus patient, said her organization is thrilled about the medication’s approval.
“There is now a new treatment for this debilitating and life-diminishing condition that is four times higher for people of African descent and Asians and two times higher for Hispanics/Latinos and Native Americans.
At a time when our nation faces extreme challenges such as addressing and overcoming social inequities and health disparities, this is welcome and promising news, especially since both lupus nephritis and COVID-19 disproportionately impact communities of color.”
INFORMATION CREDIT: The following medical information was produced by Barbara Brody (Creakyjoints.org)
IMAGE CREDIT: Center for Disease Control
NOTE: The following material has been reproduced, strictly for educational and illustrative purposes, related to the materials subject. RLS Media does not endorse or confirm the effectiveness of any product or study conducted in the searcher and studies of any product in the article.
